Silicon Briquette,Silicon Slag Briquette,Silicon Carbide Briquette,Silicon Alloy Briquettes YINCHUAN BINHE ABRASIVES CO.,LTD. , http://www.nxbhzhang.com
Graphene is a "single-layer graphite sheet" which is a basic structural unit constituting graphite; and carbon nanotubes are a cylindrical structure in which graphene is crimped (Fig. 1). As representatives of one-dimensional (1D) and two-dimensional (2D) nanomaterials, they are complementary in structure and performance. From the structural point of view, carbon nanotubes are one-dimensional crystal structures of carbon; while graphene is composed of only a single carbon atom layer, it is a true two-dimensional crystal structure. From a performance point of view, graphene has properties comparable to or superior to carbon nanotubes, such as high electrical conductivity and thermal conductivity, high carrier mobility, free electron mobility, high strength, and stiffness. At present, research on carbon nanotubes has reached a certain depth and breadth in terms of preparation technology, performance characterization and application exploration. The close relationship between composition and structure makes the two have many similarities in research methods. In fact, many studies on graphene were first carried out inspired by carbon nanotube related research. 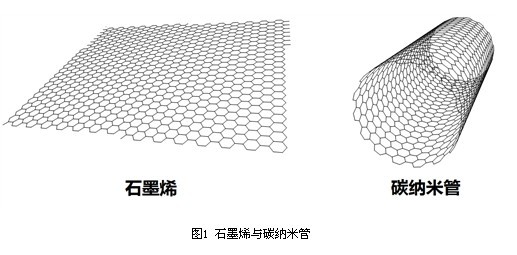 The development of graphene is very similar to that of carbon nanotubes. Before the discovery of carbon nanotubes, there are three main crystal structures of carbon: graphite, diamond and fullerenes (represented by C60 [2]). At that time, carbon fiber (industrial application) and carbon nanofibers had been fully studied; after the discovery of carbon nanotubes [3], people began to pay attention to the similarities and differences between carbon nanotubes and carbon nanofibers. On the surface, in the crystal structure, the degree of crystallization of carbon nanofibers is relatively poor, and there are many defects. The graphite layer is discontinuously arranged and has a large diameter, which does not really belong to the crystal structure of carbon, or is only graphite. a derivative. From this point of view alone, the appearance of carbon nanotubes seems to be only an extension of carbon nanofibers. Therefore, many people do not attribute the discovery of carbon nanotubes to Sumio Iijima. In fact, the discovery of carbon nanotubes is reflected in the renewal of human concepts, marking a deeper understanding of the carbon crystal structure (and even the entire carbon paradigm), which is a major step forward in essence. In particular, the controllable synthesis of single-walled carbon nanotubes, double-walled and thin-walled carbon nanotubes has laid a solid experimental foundation for fully understanding the properties of carbon nanotubes. It is worth mentioning that some methods for preparing carbon nanotubes are mostly derived from carbon nanofibers (such as chemical vapor deposition). In some early studies, the distinction between carbon nanofibers and carbon nanotubes was not very strict. Comparing graphene with carbon nanotubes (Fig. 2), a similar development trajectory is evident. Before the discovery of graphene in the experiment, some tiny graphite grains, whiskers or graphite sheets (more layers) have been synthesized and widely studied. Expanded graphite is also based on the concept of exfoliated graphite. The related technologies are well developed and industrialized for a long time. Similar to the relationship between single-walled, double-walled, thin-walled carbon nanotubes, except for the graphene (single layer) in the strict sense, the double layer and a few layers of graphite layer are also clearly distinguished from the block in structure and performance. Bulk graphite is also classified as a graphene in a broad sense.
The development of graphene is very similar to that of carbon nanotubes. Before the discovery of carbon nanotubes, there are three main crystal structures of carbon: graphite, diamond and fullerenes (represented by C60 [2]). At that time, carbon fiber (industrial application) and carbon nanofibers had been fully studied; after the discovery of carbon nanotubes [3], people began to pay attention to the similarities and differences between carbon nanotubes and carbon nanofibers. On the surface, in the crystal structure, the degree of crystallization of carbon nanofibers is relatively poor, and there are many defects. The graphite layer is discontinuously arranged and has a large diameter, which does not really belong to the crystal structure of carbon, or is only graphite. a derivative. From this point of view alone, the appearance of carbon nanotubes seems to be only an extension of carbon nanofibers. Therefore, many people do not attribute the discovery of carbon nanotubes to Sumio Iijima. In fact, the discovery of carbon nanotubes is reflected in the renewal of human concepts, marking a deeper understanding of the carbon crystal structure (and even the entire carbon paradigm), which is a major step forward in essence. In particular, the controllable synthesis of single-walled carbon nanotubes, double-walled and thin-walled carbon nanotubes has laid a solid experimental foundation for fully understanding the properties of carbon nanotubes. It is worth mentioning that some methods for preparing carbon nanotubes are mostly derived from carbon nanofibers (such as chemical vapor deposition). In some early studies, the distinction between carbon nanofibers and carbon nanotubes was not very strict. Comparing graphene with carbon nanotubes (Fig. 2), a similar development trajectory is evident. Before the discovery of graphene in the experiment, some tiny graphite grains, whiskers or graphite sheets (more layers) have been synthesized and widely studied. Expanded graphite is also based on the concept of exfoliated graphite. The related technologies are well developed and industrialized for a long time. Similar to the relationship between single-walled, double-walled, thin-walled carbon nanotubes, except for the graphene (single layer) in the strict sense, the double layer and a few layers of graphite layer are also clearly distinguished from the block in structure and performance. Bulk graphite is also classified as a graphene in a broad sense. 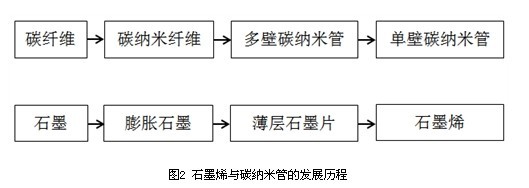 Although graphene and carbon nanotubes have similar predecessors, they are likely to have a different future. There are many reasons for this, but it can ultimately be attributed to the dispute between one-dimensional materials and two-dimensional materials. Nanowires and nanotubes are often at a disadvantage in the competition with thin film materials. Taking carbon nanotubes as an example, a single carbon nanotube can be regarded as a single crystal with a high aspect ratio, but current synthesis and assembly techniques have not been able to obtain carbon nanotube crystals with macroscopic dimensions (will be described later). In detail), thereby limiting the application of carbon nanotubes. The advantage of graphene is that it is a two-dimensional crystal structure, which can realize continuous growth of large area, and combines Bottom-up and Top-down. The future application prospect is bright. In addition, graphene is favored by physicists and is an ideal platform for scientific experiments and solving scientific problems. This is also the main factor that contributed to this award. The status of carbon nanotubes is ambiguous, it is impossible to give the physics prize, and it is not like giving a chemistry prize, let alone the C60 is the first.
Although graphene and carbon nanotubes have similar predecessors, they are likely to have a different future. There are many reasons for this, but it can ultimately be attributed to the dispute between one-dimensional materials and two-dimensional materials. Nanowires and nanotubes are often at a disadvantage in the competition with thin film materials. Taking carbon nanotubes as an example, a single carbon nanotube can be regarded as a single crystal with a high aspect ratio, but current synthesis and assembly techniques have not been able to obtain carbon nanotube crystals with macroscopic dimensions (will be described later). In detail), thereby limiting the application of carbon nanotubes. The advantage of graphene is that it is a two-dimensional crystal structure, which can realize continuous growth of large area, and combines Bottom-up and Top-down. The future application prospect is bright. In addition, graphene is favored by physicists and is an ideal platform for scientific experiments and solving scientific problems. This is also the main factor that contributed to this award. The status of carbon nanotubes is ambiguous, it is impossible to give the physics prize, and it is not like giving a chemistry prize, let alone the C60 is the first.
Post-Note: Graphene as a basic structural unit of graphite (and later carbon nanotubes) has been studied theoretically for more than 60 years [4]. Prior to the work of Geim and Novoselov, thin-layer graphite has been reported in many places. For example, Philip Kim [5] of Columbia University and Rodney Rouff [6] of the University of Texas at Austin (then at Washington University) studied graphite stripped graphite sheets, but unfortunately, single-layer graphene was not obtained.
Graphene and carbon nanotubes: the same predecessor, different in this world
On October 4, 2010, the Nobel Prize in Physics was announced. The winners were Andre Geim and Konstantin Novoselov of the School of Physics and Astronomy at the University of Manchester, UK. The reason for the award was “a pioneering experiment on graphene in two-dimensional space materials†. From the successful stripping of graphene in 2004 [1] to the Nobel Prize in 2010, what magical power made this seemingly “ordinary†carbon material create a legendary myth in just six years? Looking back at his brothers and sisters, they have been discovered for nearly 20 years since 1991. After years of ups and downs, they have been ups and downs, but they are "making clothes for others."